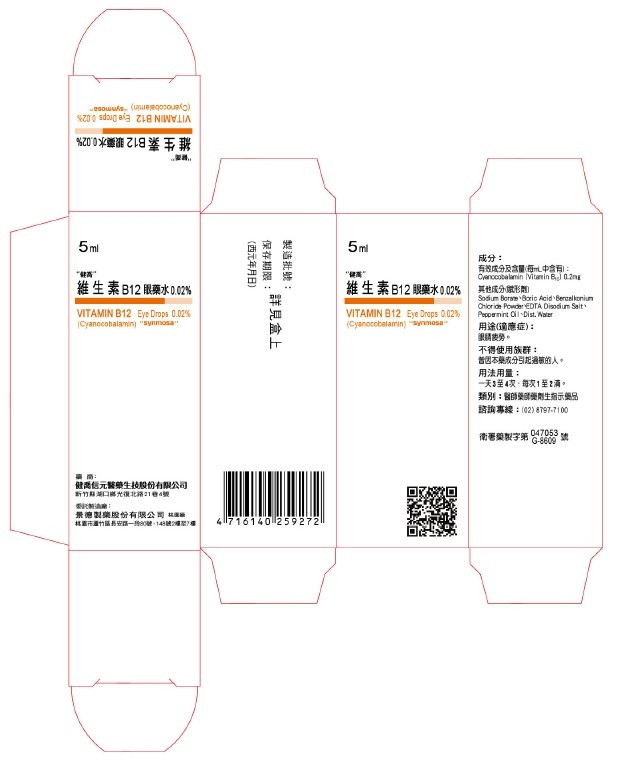
"Jian Qiao Vitamin B12 Eye Drops 0.02%".
(Photographed by reporter Wu Liangyi)
[Reporter Wu Liangyi/Taipei Report] The Food and Drug Administration issued a drug recall alert today. "Jianqiao B12 Eye Drops" was found to be insufficient in the main ingredient during the stability test, which may affect the effect of use. The Food and Drug Administration requires that in January next year A batch of 48,000 bottles was recovered 7 days ago.
The main ingredient of "Jianqiao Vitamin B12 Eye Drops 0.02%" is CYANOCOBALAMINE (1% SPRAY DRIED), which is mainly used for eye fatigue.
Due to the notification of defective drugs, the Food and Drug Administration requested to start the recall. The main component content should be between 95% and 115%. However, during the stability test, it was found that the main component content of this batch of drugs was slightly lower than 95%. So start recycling.
Please read on...
Hong Guodeng, section chief of the drug group of the Food and Drug Administration, said that the batch size is 48,000 bottles, and the main ingredients are slightly low so that they will not pose risks to human health.
The manufacturer must complete the recycling operation before January 7 next year, and should submit the recycling results report and follow-up preventive and corrective measures.
☆Health news will never be missed, click like to follow the fan page.
☆For more important medical news, please go to Liberty Health.com.
keywords
eye drops
drug recovery
eye fatigue
drug recovery
related news